lostdaytomorrow said:
I would like to hear more about this! I know scottwax has used it for a very long time successfully. I can't imagine it causing any serious issues being cut 20:1 though.
A little understanding about the chemistry of leather cleaning, do a little testing and draw your own conclusion from, should set your taught straight.
Leather is amphoteric, at the molecular level, any pH value solution above its iso-electric point will cause the protein fiber to shift ionic negative and any solution below its iso-electric point (the pH neutral of leather is between 3 and 5 is also the iso-electric point of the leather) will strengthen the leather ionic positive.
Leather is not all 100% protein fiber; it has its other constituents especially the tanning agents that convert the perishable protein fiber to non-perishable leather. Tanning alone does not impart enough softness to the leather. The suppleness and strength of the leather depends on the fatliquor (ionic charged fat, oil and water). The tanning agent and the fatliquor are hydrogen bonded to the protein fiber with forces of ionic attraction similar to a magnet – like poles repel and unlike poles attracts. When the ionic charges between the protein fiber and the fatliquor weaken, the leather begins to denature with signs of dryness, shrinkage, stiffness that leads to cracks.
The problem with leather is that the protein fiber is amphoteric and should remain ionic positive (+ve), while the other chemistry remains unchanged ionic negative (-ve). It is this shifting of the protein fiber to ionic negative (-ve) with alkaline solution that is the cause of the problem with leather.
An acidic cleaning solution, therefore charges the protein fiber more ionic positive (+ve), thus strengthen the leather chemistry integrity of the leather with the other ionic (-ve) tanning agent and fatliquor.
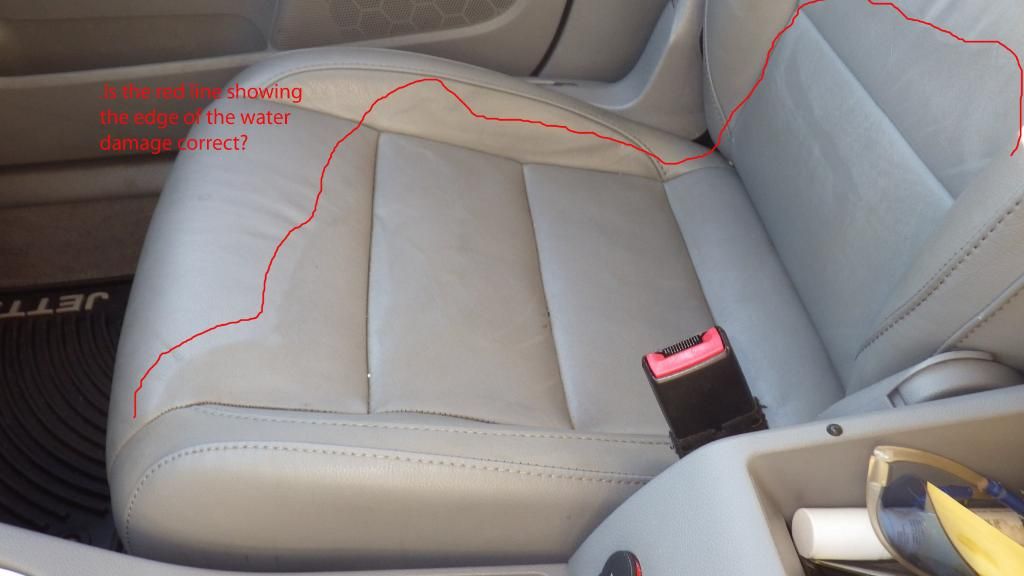
An alkaline cleaning solution (“woollike”), shift the protein fiber ionic (-ve), thus repels the hydrogen bond between the protein fiber and especially the ionic negative (-ve) fatliquor (as seen in the picture below where rainwater alone at almost pH 7 will cause the protein fiber to shift ionic negative (-ve) thus shrink and stiffen the leather).
In most pigmented auto leathers, which often is non-absorbent we are concern about the stitching holes and the perforated holes or when micro-crazing develops, there is when the leather structure are exposed to absorption of cleaning solution.
If rainwater with a pH value close to 7 can do damages to leathers, any cleaning solution higher than that of water will eventually damage the leather especially those above the pH 8 range (woollike at 1: 20 dilution reads from pH 8 to 9).
#1 Water (pH 6 to 7) Damaged Areas
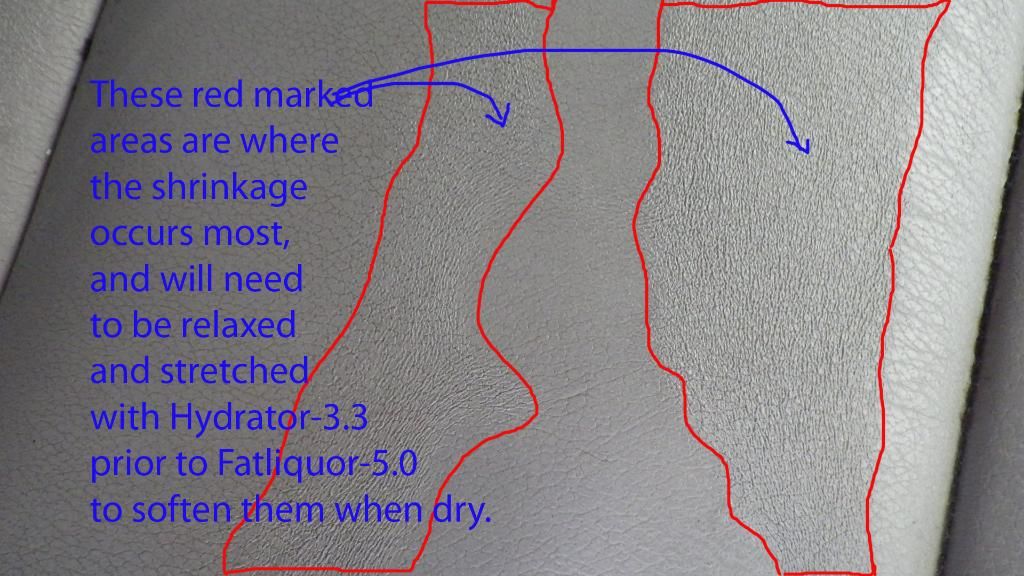
#2 Close-up view of the leather Shrinkage that cause the Stiffness.
Leather rejuvenating technique . . .
#1 Fatliquor-5.0 is applied to the cleaned surface and after Hydrator-3.3
#2 A paper rag/towel is used as a reservoir to contain the fatliquor and is covered with a plastic cling wrapper to control evaporation
What do you think?
Alkaline cleaning solution damages leather.
Acidic cleaning solution strengthens the chemistry integrity of leather.
True or False, you vote?
Roger Koh
[email protected]