David Fermani
Forza Auto Salon
What's the expert concensus on using steam to clean leather? Pros / Cons??
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
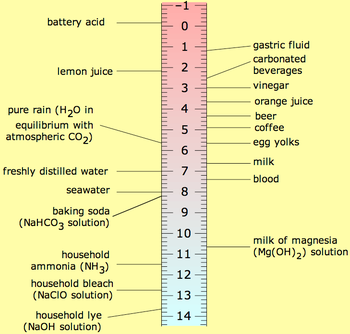
TortoiseAWD said:What in the name of science are you talking about? Pure water has a pH of 7 *by definition*. If you're not talking about pure, neutral water, then say so instead of being vague. You're either a) not talking about pure water, b) unclear on what pH means, or c) doing a tap-dance to say that a leather-safe solution should have a pH of 3 - 5, in which case you're no longer talking about pure water.
pH of pure water
pH Definition
Why is pH = 7 the neutral point?
A pH of 3 - 5 is roughly equivalent to the acidity of orange juice. I'm no chemist, but I do recall my high school science.
Tort
Roger Koh said:---------------
By the way where do you get your pure water?
Have you ever take a pH reading of "your pure water" yourself?
Instead of passing on secondhand information, which is not yours!
We are professional that practice not just talk!
SuperBee364 said:Hmmm.. There's not really any need to ask questions about a scientific fact that has been known for a long, long time: the Ph of pure water is 7.0.
Muddying the waters (pardon the word play)with questions about where you get your pure water from is not at all relevant. What *is* relevant is that the Ph of pure water is 7.
SuperBee364 said:Any water that doesn't test at 7.0 Ph is not pure.
TortoiseAWD said:This is like someone arguing that 2 + 2 = 5 . . . for sufficiently large values of "2", of course.
Where do you get your water, pure or not? If it has a pH of 3 - 5 coming out of the tap, your municipality has some real problems with water treatment.
If you want to argue that a safe cleaning solution for leather (NOT pure water) has a pH in that range, I'll take your word for it; I'm not a "leather chemist".
Roger Koh said:----
Water can be so… good!
Water can be so… bad!
It depends on the pH of the water!
It also depends on its purity or the solids that it carries!
So, a leather-safe pure water should have a pH value of 3 - 5.
Roger Koh
Leather Doctor®
TortoiseAWD said:This is like someone arguing that 2 + 2 = 5 . . . for sufficiently large values of "2", of course.
JuneBug said:Can we all agree that there are differences in "professional" opinion and it's up to you , the consumer to make a choice.
Dsoto87 said:Man this thread is really out to confuse people. First they say condition it with oil. Then they say condition it with water. AND NOW you got people trying to reinvent the PH scale.
Roger is an EXPERT PROFESSIONAL though! If he says 3-3.5=pure water than, damnit, that's pure neutral water.
