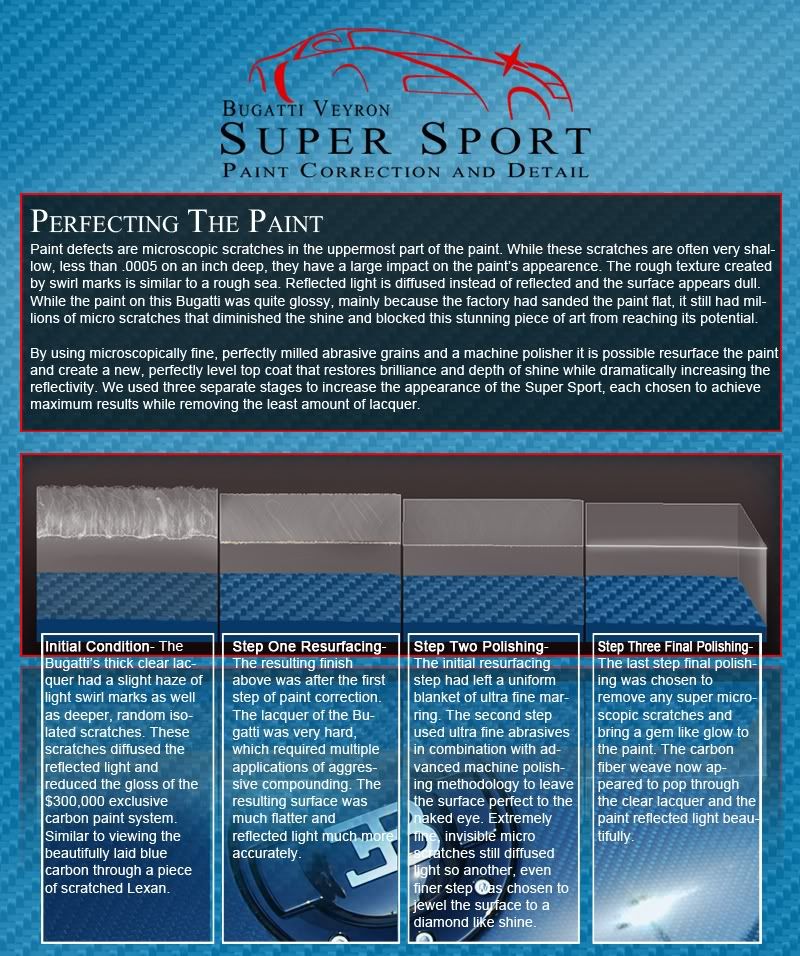
by David W. Bynon
<font size="1">Copyright©, 1999-2002, Autopia Car Care -- All Rights Reserved</font>
Some very well intentioned car enthusiasts use one cleaner as a general purpose tool for detailing. I often hear people say they use a product like Simple Green or an engine degreaser to clean everything from wheels to carpets. In my opinion, this is both dangerous and harmful to the car. After all, none of us would bathe with laundry detergent, so why would we treat delicate car surfaces with harsh chemicals?
Basic Dirt
In the science of car detailing, dirt can be classified in three ways: organic soil, non-organic soil, and petroleum soil. Not much else exists in the car world. If you can figure out the type of soil, you can select the proper cleaner. If you use the proper cleaner, 99% of all stains will come out.
Organic soil is anything that contains carbons. This includes all proteins, animal fats, body oils, mold, yeast, bugs, bacteria, animal and bug excrement, and carbohydrates. Those chilies cheese fries you spilled on the front seat are the classic organic soil.
No-organic soil is soil that does not contain carbons. This is most common on the exterior of a car in the form of water spots. Scale and lime deposits are the most common form of non-organic soil.
Petroleum soils are substances that do not contain water and will not mix with water. Petroleum soils do not have a pH factor. Petroleum soils include common chemicals like motor oil, grease and road tar. By the way, chewing gum also falls into this category.
It is also possible to have a combination of the three soils. This is a nightmare, as combination soils are difficult to identify. How do you choose a cleaner for something you can’t identify?
Cleaning Chemicals 101
The car care industry is full of chemicals for cleaning. There is no such thing as a do-all cleaner. It simply does not exist. Detailing chemicals are mixtures of different ingredients that help them do a particular job. The most common chemical functions include: surfactants, solvents, wetting agents, saponifiers, and chelators.
The word surfactant is a fancy term for any soap or detergent. Surfactant molecules are created with two compounds. One is attracted to the soil, the other is attracted to water. The chemical compound that’s attracted to water is called a hydrophile. Its job is to surround the soil. Likewise, the chemical compound that’s attracted to soil is called a hydrophobe. Its job is to break soil into smaller pieces so it can be surrounded by the hydrophile.
Every cleaner needs a solvent to dissolve soil. The most common solvent is one you might not even think of, water. Some solvents, such as mineral spirits, work great on petroleum soils, like tar and grease, and may be necessary on surfaces that might be damaged by water. Other solvents that are common in car care chemicals include dilimoneen (made from orange and lemon peels) and butyl. Although expensive, dilimoneen is a safe solvent to use throughout the car.
Chemists modify the hydrophile and hydrophobe molecules to change the characteristics of a surfactant. For example, a surfactant that is a good detergent (detergents break a soil’s bond to a surface) will not be a good penetrating agent. Penetrating and wetting allows water to surround soil so it can be removed. So, the chemist can improve the performance of a good detergent surfactant by including a second surfactant that has good wetting and penetrating qualities.
Did you know that animal fat is used in the manufacturing of soaps? Boil some pig fat, add a handful of lye, and you can make a soap bar. The same chemical process that makes soap can be used to remove fats and oils. Chemists use agents called saponifiers (basically a strong alkaline substance) to convert fats and oils into soap. Once transformed to soap, fats and oils can be washed away with water.Â
Have you ever noticed how much better soap does in soft water? Hard water, which is any water that contains high concentrations calcium, iron, magnesium and other minerals, thwart the cleaning ability of a chemical. The cleaner sees the minerals in hard water as soil, which uses up the cleaning agents. To combat this problem, chemists add chelating agents to their cleaners to bind the minerals so the cleaner can go after the real soil. This is why some car wash shampoos seem to hold the suds longer than others.
The Importance of Understanding pH
The term “pH� is a measurement of the relationship between hydrogen ions and hydroxyl ions. When you have more hydrogen ions than hydroxyl ions, you have an acid. Likewise, if you have more hydroxyl ions than hydrogen ions you have an alkaline.
Any solution with a water base has a pH measurement. The pH scale runs from 1 to 14. The first half of the scale (1.0 to 6.9) represents acids, and the second half (7.1 to 14.0) represents alkaline. Pure water is neutral and has a pH of 7.0. If a substance does not contain water, such as mineral spirits, it does not have a pH.
If you know the pH of a cleaner you will know if it is an acid or an alkaline. This will help you know where to use the cleaner. For example, a carpet shampoo would have a pH around 8 or 9, whereas a wheel cleaner would be between 12 and 14. Use the wheel cleaner on your carpet, and you’ll have a real mess.
Now You Know
Now that you know the basics, here are some tips to remember when selecting a cleaner:
* Organic soil is cleaned with alkaline cleaners
* Non-organic soil is removed with acids
* Petroleum soil must be removed with a petroleum-based cleaner
* Never use a cleaner that’s overly aggressive for the soil
* When in doubt, test the chemical on a hidden part of the surface to verify compatibility
<font size="1">Copyright©, 1999-2002, Autopia Car Care -- All Rights Reserved</font>
Some very well intentioned car enthusiasts use one cleaner as a general purpose tool for detailing. I often hear people say they use a product like Simple Green or an engine degreaser to clean everything from wheels to carpets. In my opinion, this is both dangerous and harmful to the car. After all, none of us would bathe with laundry detergent, so why would we treat delicate car surfaces with harsh chemicals?
Basic Dirt
In the science of car detailing, dirt can be classified in three ways: organic soil, non-organic soil, and petroleum soil. Not much else exists in the car world. If you can figure out the type of soil, you can select the proper cleaner. If you use the proper cleaner, 99% of all stains will come out.
Organic soil is anything that contains carbons. This includes all proteins, animal fats, body oils, mold, yeast, bugs, bacteria, animal and bug excrement, and carbohydrates. Those chilies cheese fries you spilled on the front seat are the classic organic soil.
No-organic soil is soil that does not contain carbons. This is most common on the exterior of a car in the form of water spots. Scale and lime deposits are the most common form of non-organic soil.
Petroleum soils are substances that do not contain water and will not mix with water. Petroleum soils do not have a pH factor. Petroleum soils include common chemicals like motor oil, grease and road tar. By the way, chewing gum also falls into this category.
It is also possible to have a combination of the three soils. This is a nightmare, as combination soils are difficult to identify. How do you choose a cleaner for something you can’t identify?
Cleaning Chemicals 101
The car care industry is full of chemicals for cleaning. There is no such thing as a do-all cleaner. It simply does not exist. Detailing chemicals are mixtures of different ingredients that help them do a particular job. The most common chemical functions include: surfactants, solvents, wetting agents, saponifiers, and chelators.
The word surfactant is a fancy term for any soap or detergent. Surfactant molecules are created with two compounds. One is attracted to the soil, the other is attracted to water. The chemical compound that’s attracted to water is called a hydrophile. Its job is to surround the soil. Likewise, the chemical compound that’s attracted to soil is called a hydrophobe. Its job is to break soil into smaller pieces so it can be surrounded by the hydrophile.
Every cleaner needs a solvent to dissolve soil. The most common solvent is one you might not even think of, water. Some solvents, such as mineral spirits, work great on petroleum soils, like tar and grease, and may be necessary on surfaces that might be damaged by water. Other solvents that are common in car care chemicals include dilimoneen (made from orange and lemon peels) and butyl. Although expensive, dilimoneen is a safe solvent to use throughout the car.
Chemists modify the hydrophile and hydrophobe molecules to change the characteristics of a surfactant. For example, a surfactant that is a good detergent (detergents break a soil’s bond to a surface) will not be a good penetrating agent. Penetrating and wetting allows water to surround soil so it can be removed. So, the chemist can improve the performance of a good detergent surfactant by including a second surfactant that has good wetting and penetrating qualities.
Did you know that animal fat is used in the manufacturing of soaps? Boil some pig fat, add a handful of lye, and you can make a soap bar. The same chemical process that makes soap can be used to remove fats and oils. Chemists use agents called saponifiers (basically a strong alkaline substance) to convert fats and oils into soap. Once transformed to soap, fats and oils can be washed away with water.Â
Have you ever noticed how much better soap does in soft water? Hard water, which is any water that contains high concentrations calcium, iron, magnesium and other minerals, thwart the cleaning ability of a chemical. The cleaner sees the minerals in hard water as soil, which uses up the cleaning agents. To combat this problem, chemists add chelating agents to their cleaners to bind the minerals so the cleaner can go after the real soil. This is why some car wash shampoos seem to hold the suds longer than others.
The Importance of Understanding pH
The term “pH� is a measurement of the relationship between hydrogen ions and hydroxyl ions. When you have more hydrogen ions than hydroxyl ions, you have an acid. Likewise, if you have more hydroxyl ions than hydrogen ions you have an alkaline.
Any solution with a water base has a pH measurement. The pH scale runs from 1 to 14. The first half of the scale (1.0 to 6.9) represents acids, and the second half (7.1 to 14.0) represents alkaline. Pure water is neutral and has a pH of 7.0. If a substance does not contain water, such as mineral spirits, it does not have a pH.
If you know the pH of a cleaner you will know if it is an acid or an alkaline. This will help you know where to use the cleaner. For example, a carpet shampoo would have a pH around 8 or 9, whereas a wheel cleaner would be between 12 and 14. Use the wheel cleaner on your carpet, and you’ll have a real mess.
Now You Know
Now that you know the basics, here are some tips to remember when selecting a cleaner:
* Organic soil is cleaned with alkaline cleaners
* Non-organic soil is removed with acids
* Petroleum soil must be removed with a petroleum-based cleaner
* Never use a cleaner that’s overly aggressive for the soil
* When in doubt, test the chemical on a hidden part of the surface to verify compatibility