[
The term pH is a measurement of the relationship between hydrogen ions and hydroxyl ions. When you have more hydrogen ions than hydroxyl ions, you have an acid. Likewise, if you have more hydroxyl ions than hydrogen ions you have a base (alkali).
The pH scale is a measure of the acidity or basicity (Alkali) of a solution. It is approximates but is not equal to p [H], the negative logarithm base 10) Base (Acid) 1-7, Alkaline 7- 14; the pH of a solution is temperature-dependent.
Unfortunately the pH scale is logarithmic; for every integer that the scale decreases the material is 10 times more acidic. Those of us in earthquake country know all too well the consequences of a change of from 6 to 7 on the logarithmic, Richter scale. The difference in the pH scale is just as dramatic and therefore just as misleading.
Base
Alkalis neutralize acids, and solutions of alkalis are soapy to the touch. The strength of an alkali is measured by its hydrogen-ion concentration, indicated by the pH value.
They may be divided into strong and weak alkalis: a strong alkali (for example, potassium hydroxide, KOH) ionizes completely when dissolved in water, whereas a weak alkali (for example, ammonium hydroxide, NH4OH) exists in a partially ionized state in solution. All alkalis have a pH above 7.0.
The hydroxides of metals are alkalis. Those of sodium and potassium are corrosive; both were historically derived from the ashes of plants.
The four main alkalis are-
1. Sodium hydroxide (caustic soda, NaOH)
2. Potassium hydroxide (caustic potash, KOH)
3. Hydroxide calcium (slaked lime or limewater, Ca (OH) 2)
4. Aqueous ammonia (NH3 (aq)). Their solutions all contain the hydroxide ion OH-, which gives them a characteristic set of properties
If the affected paintwork is not neutralized any remaining acid residue will be reactivated each time it comes into contact with moisture and heat. Water contains 2- hydrogen and 1-oxygen atom and will acts as a catalyst and a carrier system for acid. Oxygen is an oxidizer; ozone is an allotropic form of oxygen (an oxidizer is any component that emits oxygen); many chemical compounds react to slight heating and an oxidizing process.
Acid
Strong acids include the heavier hydrophilic acids: however, Hydrofluoric Acid (HF) is relatively weak. Acids are acids by virtue of the presence of an excess of hydrogen ions in the solution, Their salts are created when the positive hydrogen ions are replaced with positive metal ions, for example when Hydrochloric Acid (HCL) reacts with Sodium (Na) to produce NaCl with the release of H2 gas.
1. Hydrochloric Acid (HCI): or Muriatic Acid, its historical but still occasionally used name, this is a highly corrosive acid (pH of minus 1) and is often used to clean calcium carbonate build up from the inside of kettles or from around water faucets and from shower heads
2. Sulphuric Acid (H2 SO4): this is a common acid in both the laboratory and industry. It is both highly corrosive and economical to manufacture, which makes it the reagent of choice for many applications;
3. Phosphoric Acid (H3PO4): this acid is used to remove rust and rust stains from metal tools and from car bodies undergoing repairs;
4. Nitric Acid (HNO): this is another common laboratory acid used as a reagent in many chemical tests and experiments due to the fact that almost all of its products (salts) are soluble in water;
5. Hydrofluoric Acid (HF): This acid is extremely corrosive and has the unique property of being able to etch (eat away) glass. Consequently it is used in industry to write signs on glass windows in stores and office buildings or on glass products.
Reactivity
Acid is inert, but add moisture (dew, rain, car washing etc) so now you have an acid + water +oxygen + ozone; add heat to this equation (reactivity) all of which equates to a highly concentrated acidic solution, which causes a concave indentation (acid etching) to the paint surface. This must be neutralized to stop the ongoing reaction process as moisture acts as a catalyst and a carrier system for acid, which will permeate the paint system matrix.
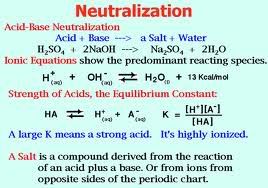
Neutralization
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula NaHCO3Many laboratories keep a bottle of sodium bicarbonate powder within easy reach, because sodium bicarbonate is amphoteric, reacting with acids and bases. Furthermore, as it is relatively innocuous in most situations, there is no harm in using excess sodium bicarbonate
Surface preparation - wash and then clean the paint surface by using a chemical paint cleaner (Klasse all in one (AIO) or ValuGuard "N" New Car Prep
Acid - ValuGuard Acid Neutralizer (Step I) - diluted 1:8 with distilled water it neutralizes acids deposited on the paint surface and in the micro-pores of the paint
Base (Alkali)- ValuGuard Alkaline Neutralizer (Step II) - deep-cleans painted surfaces to remove alkaline deposits
Acid / Alkali Etching Removal â
1. First clean the paint surface and then neutralize the acid or alkali
2. Use a machine polish (Optimum Polish, Optimum Compound) and a cutting (LC White, Orange or Yellow) foam pad (speed # 4- 5.0 or 1200 RPM) to level the surface
3. For PPG CeramiClear⢠Clear Coat or other hard clear coats substitute Menzerna for machine polish; i.e. PO 203 S - Power Finish
4. Use the least aggressive polish/foam pad first, if this doesnât remove the problem step-up to a more aggressive polish / foam pad set-up
5. If none of the above remove the etching use a wet-sanding process with 2000, 2500 and then 3000 (or 4000) grit finishing paper
Don`t Play Mad Scientist
Just remember that anytime two chemicals with completely different formulas and / or functions are combined, at best the effectiveness of each chemical is reduced, and/or the chemicals may not be compatible
Don`t haphazardly mix chemicals; pay attention to the order in which chemicals are to be added to each other and do not deviate from the instructions. Even chemicals that mix to produce seemingly safe products should be handled carefully. For example, hydrochloric acid and sodium hydroxide will give you salt water, but the reaction could break your glassware or splash the reactants onto you if you aren`t careful.
Some rules are NOT made to be broken. That is true of the rules used for chemicals. They are established for your safety and those of otherâs
Results 1 to 1 of 1
-
03-19-2012, 09:32 AM #1What gets overlooked too often is that one must be a student before becoming a teacher.Post Thanks / Like - 1 Likes, 0 Thanks, 0 Dislikes
 dwaleke liked this post
dwaleke liked this post
Thread Information
Users Browsing this Thread
There are currently 1 users browsing this thread. (0 members and 1 guests)
Similar Threads
-
Removal of Acidic / Alkali Wheel Surface Damage
By togwt in forum Autopia Detailing WikiReplies: 0Last Post: 08-10-2012, 02:39 PM -
Valugard A-B-C Neutralization Procedure Video
By David Fermani in forum Car Detailing Product DiscussionReplies: 9Last Post: 05-17-2012, 05:18 PM -
Mixing oil base with water base..
By Al Buff in forum Car Detailing Product DiscussionReplies: 2Last Post: 11-16-2007, 08:40 AM -
solvent base vs. water base
By airjames in forum Car DetailingReplies: 18Last Post: 01-10-2003, 11:35 PM -
Autoint`s neutralization system
By AlphaSpread in forum Car DetailingReplies: 2Last Post: 01-01-1970, 12:00 AM






 Reply With Quote
Reply With Quote
Bookmarks